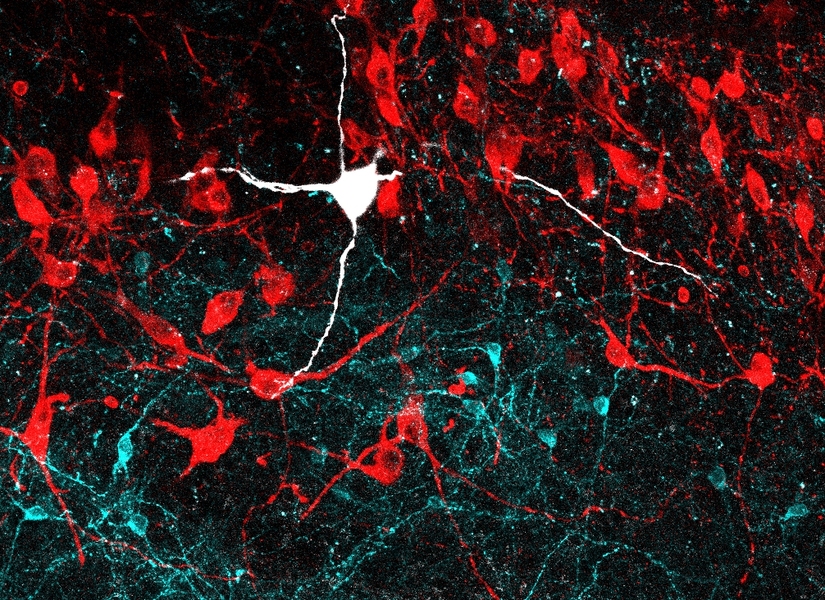
Studies suggest the oscillations created by nerve cell activity have roles of their own.
We can’t see it, but brains hum with electrical activity. Brain waves created by the coordinated firing of huge collections of nerve cells pinball around the brain. The waves can ricochet from the front of the brain to the back, or from deep structures all the way to the scalp and then back again.
Called neuronal oscillations, these signals are known to accompany certain mental states. Quiet alpha waves ripple soothingly across the brains of meditating monks. Beta waves rise and fall during intense conversational turns. Fast gamma waves accompany sharp insights. Sluggish delta rhythms lull deep sleepers, while dreamers shift into slightly quicker theta rhythms.
Researchers have long argued over whether these waves have purpose, and what those purposes might be. Some scientists see waves as inevitable but useless by-products of the signals that really matter — messages sent by individual nerve cells. Waves are simply a consequence of collective neural behavior, and nothing more, that view holds. But a growing body of evidence suggests just the opposite: Instead of by-products of important signals, brain waves are key to how the brain operates, routing information among far-flung brain regions that need to work together.
MIT’s Earl Miller is among the neuroscientists amassing evidence that waves are an essential part of how the brain operates. Brain oscillations deftly route information in a way that allows the brain to choose which signals in the world to pay attention to and which to ignore, his recent studies suggest.
Other research supports this view, too. Studies on people with electrodes implanted in their brains suggest brain waves, and their interactions, help enable emotion, language, vision and more.
When these waves are abnormal, brainpower suffers, studies find. Detailed looks at how the brain uses these waves raise the possibility of tweaking the signals with electrical nudges — interventions that could lead to therapies that can correct memory problems and mental illness, for instance. Already, early attempts have led to improvements in people’s memory.
Types of brain waves
Scientists are studying how oscillations generated by nerve cells affect brain function. Although the boundaries between different wave types can be fuzzy, these oscillations can be grouped by frequency.
Fast gamma waves have been linked to states of high attention. 30 to 80 Hz
Beta waves may be involved in movement and complex tasks such as memory and decision making. 12 to 30 Hz
The first neuronal oscillations discovered, alphawaves appear when a relaxed person closes his eyes. 8 to 12 Hz
Theta oscillations may help the brain sort information essential for navigation. 4 to 8 Hz
Slow delta waves mark deep sleep and anesthesia. 1.5 to 4 Hz
C. CHANG
These insights about brain waves coincide with a shift in neuroscience away from a view that reduces the brain down to the behavior of single nerve cells, or neurons. That’s like thinking of the brain as “a giant clock, and if you figure out each gear, you’ll figure out the brain,” Miller says. But “it’s not just individual neurons in a giant clock. It’s networks interacting in a very dynamic, fluid way.”
Central to those interactions, Miller and others think, are coordinated brain waves. “The oscillations are the most powerful signal in the brain,” Miller says. “How could evolution not have taken advantage of that?”
In three recent papers, Miller and colleagues argue that two different types of brain waves — beta and gamma — work together to selectively choose the information that makes it into working memory. Gamma waves that cycle 30 to 80 times per second (30 to 80 hertz) help coordinate information streaming in from our senses — what we feel, see and smell. In contrast, slower 12 to 30 Hz beta waves are the messages that help keep us on task by guiding the brain toward the sensory signals worth paying attention to.
These two types of brain oscillations engage in a neural seesaw: When beta waves are strong, akin to a stereo blasting, gamma waves are weak, as if the volume had been dialed down, and vice versa. Miller and colleagues saw this push-and-pull action in the brains of monkeys with implanted electrodes as the animals completed a tricky memory task, one that required the monkeys to hold several pieces of information in their minds at the same time. The results were described January 26 in Nature Communications. “At all these complex decision points, you can see the beta and gamma doing this complex dance in a way that you’d expect if they’re controlling working memory,” Miller says.
These two types of waves were generated in different parts of the brain, offering spatial clues about how the brain focuses itself, the researchers also found. Sensory information, organized by gamma waves, skims the superficial layers of the brain, experiments on monkeys showed. But slower, more goal-directed waves, a mix of alpha and beta waves, are deeper in the brain. And those slower, deeper waves could actually dial down the strength of the gamma waves that rippled along the outer brain. The deeper waves were selecting which sensory information to pay attention to, the researchers proposed in the Jan. 30 Proceedings of the National Academy of Sciences.
A third paper, in the Feb. 7 Neuron, shows similar interactions between gamma and beta waves while monkeys matched patterns of dots on a computer screen. Some of the patterns were clearly different but still belonged to the same category, an easy task akin to knowing that both a dog and a cat are types of animals. Other times, the patterns were harder to classify and required more sophisticated mental work, similar to knowing that trains and bicycles are both types of transportation. Gamma waves were present when the monkeys were puzzling out an easy category. But when higher-level categorization was required, beta waves started to roll.
These interactions between gamma and beta waves might be how the brain solves an information overload problem, Miller suspects. Incoming sensory input constantly bombards the brain, and much of it is meaningless. The brain needs a way to figure out if it should ignore the feeling of a scratchy shirt, but pay attention to the ringing phone. These two rhythms may offer a way for “volitional control over what you think about,” Miller says, allowing a person to consciously choose what information to bring to mind.
Oscillations may also shape visual information as it travels through the brain, says Charles Schroeder, a neuroscientist at the Nathan S. Kline Institute for Psychiatric Research in Orangeburg, N.Y. He and colleagues are studying a different ebb and flow of oscillations from the one Miller recently described. This one probably involves a host of different kinds of waves, including theta waves, and happens in the split second when your eyes dwell on a scene — a pause that usually lasts about 200 milliseconds.
When you look at a scene, the first half of the time it takes to stare is spent on visual information streaming into your brain. But toward the end of that fixation time, “the information flow reverses,” Schroeder says. Different neuronal oscillations carry signals from the brain’s command center, ready to direct the eyes to the next spot. “Literally within a tenth of a second before you move your eyes, there is this incredible flash of network activity in the front of the brain, and then the eyes move,” Schroeder says. “It’s really a dramatic thing.” Schroeder and colleagues have caught this action in monkeys’ brains, and more recently, in people implanted with electrodes as part of epilepsy treatment.
But some vision researchers still dismiss these oscillations as noise, convinced that the activity of single neurons — and not the collective waves that result from that activity — is the key to understanding the brain, Schroeder says. “It’s still difficult to convince people that brain oscillations are functional.”
Researchers may argue over the function of brain waves for years to come, says neuroscientist and neurologist Robert Knight of the University of California, Berkeley. He believes that information, at its core, is held in the signals zipped off by neurons. But work from his lab has convinced him that oscillations help those signals reach the right spot, connecting brain areas in important ways. “You’ve got to have a way to get brain areas communicating,” he says. “What oscillations do is provide a routing mechanism.”
And oscillations do this quickly, he says. Human brains are incredibly fast. “We’re handling massive amounts of information in subsecond time periods,” Knight says. “And you have to have some way to shape it, to control it.” Waves, he says, give the brain a way to tune out extraneous information by temporarily shutting down unnecessary communication lines.
Knight and colleagues recently spotted fast gamma waves at work as people did a wide range of tasks, including repeating words, answering questions about themselves and distinguishing male faces from female. A certain gamma wave pattern seemed to predict when people would get the right answer on these tasks, the team reported in December 2017 in Nature Human Behavior. Gamma waves, the team suspects, link up areas of the brain that are needed to turn goals into action.
If oscillations are crucial information routers in the brain, then changing them might be beneficial when information is distorted or lost. Altered oscillations have been observed in disorders such as autism, Parkinson’s disease, depression and anxiety, and even in normal aging.
A study published February 6 in Nature Communications hints at the potential of tweaking these rhythms. Youssef Ezzyat, a neuroscientist at the University of Pennsylvania, and colleagues studied memory abilities in 25 people who had electrodes implanted in their brains as part of their epilepsy treatment.
As the researchers gave the people lists of words to remember, electrodes monitored neural oscillations. A computer algorithm then figured out which assortment of brain waves indicated when a person was likely to remember the word, an assortment that varied slightly from person to person.
When those good performance signals were missing, the researchers delivered a short burst of electricity to the brain — “a bit of a nudge to course correct,” Ezzyat says. And these nudges improved performance.
Specifically manipulating brain waves to treat brains is a long way off, Ezzyat cautions. But he and colleagues are making progress. In the meantime, his results and others’ are powerful signs that brain waves aren’t just an idle hum.
Citations